China Drug Master File (DMF) Registration: A Snapshot on Pathways and Requirements
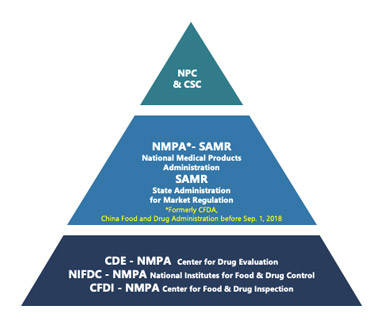
This is the first article in a series focusing on China Drug Master File (DMF) filing regulatory regulations and recent updates in the Chinese market. Keywords: China DMF, API, Excipient, Packaging Material Abstract In the rapidly evolving landscape of pharmaceutical regulation in China, it is essential for companies seeking market entry to understand the intricacies […]
