A Guide to Drug Master File (DMF) Filing in China
For pharmaceutical companies aiming to enter the Chinese market, understanding the Drug Master File (DMF) process for Active Pharmaceutical Ingredients (APIs), excipients, and packaging materials
For pharmaceutical companies aiming to enter the Chinese market, understanding the Drug Master File (DMF) process for Active Pharmaceutical Ingredients (APIs), excipients, and packaging materials
This is the second article in a series focusing on China Drug Master File (DMF) filing regulatory regulations and recent updates in the Chinese market.
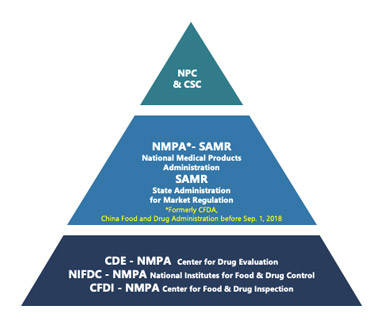
This is the first article in a series focusing on China Drug Master File (DMF) filing regulatory regulations and recent updates in the Chinese market.
Announcement No. 129 of 2023 was released by the National Medical Products Administration (NMPA) on October 13, 2023, focusing on the renewal of licenses for
China Center for Drug Evaluation (CDE) published six questions and answers on 3rd Mar. The Q&As are regarding drug master file (China DMF) filing (Q1-Q2)

Did you know that certain pharmaceutical excipients are exempt from Drug Master File (DMF) registration in China? The Chinese regulatory authority, the National Medical Products

China CDE director vows more support for drug review & approval in the coming years. The director of China’s Center for Drug Evaluation (CDE) has

NMPA News: China’s National Medical Products Administration (NMPA) has released for public comments a draft announcement on administration of registration renewal of chemical active pharmaceutical ingredients