Pathway Options for China Drug Master File (DMF) Registration:An Analysis of the Strategic Options
This is the second article in a series focusing on China Drug Master File (DMF) filing regulatory regulations and recent updates in the Chinese market. Keywords: China DMF, API, Excipient, Packaging Material, Registration Strategy Abstract This article explores the detailed pathway options for China DMF registration, focusing on APIs, excipients, and packaging materials. It highlights […]
China Drug Master File (DMF) Registration: A Snapshot on Pathways and Requirements
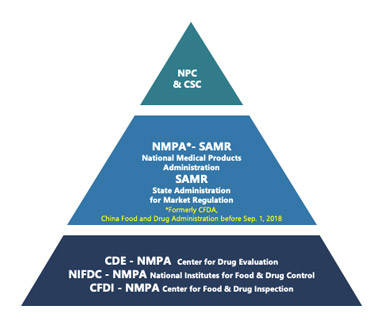
This is the first article in a series focusing on China Drug Master File (DMF) filing regulatory regulations and recent updates in the Chinese market. Keywords: China DMF, API, Excipient, Packaging Material Abstract In the rapidly evolving landscape of pharmaceutical regulation in China, it is essential for companies seeking market entry to understand the intricacies […]
China’s DMF API License Renewal: Understanding NMPA’s Policy Update
Announcement No. 129 of 2023 was released by the National Medical Products Administration (NMPA) on October 13, 2023, focusing on the renewal of licenses for Active Pharmaceutical Ingredients (APIs), referred to as API re-registration. The highlight of this update is the renewal of API licenses shall be ready at least SIX months before the old […]
China Drug Master File (DMF): Excipients Exemption List

Did you know that certain pharmaceutical excipients are exempt from Drug Master File (DMF) registration in China? The Chinese regulatory authority, the National Medical Products Administration (NMPA), has released a DMF registration exemption list for excipients. The China DMF exemption list includes excipients that are most commonly in pharmaceutical drug products which are considered by […]
CHINA CDE EYES MORE DRUG APPROVALS BY 2025

China CDE director vows more support for drug review & approval in the coming years. The director of China’s Center for Drug Evaluation (CDE) has delivered a speech on Improving the Efficiency and Quality of Drug Review & Approval at the 2022 DIA China Annual Conference, with a retrospective summary of the last 5 years’ work and […]
DMF IN CHINA NEW POLICY ON ACTIVE PHARMACEUTICAL INGREDIENTS (API) RENEWAL

NMPA News: China’s National Medical Products Administration (NMPA) has released for public comments a draft announcement on administration of registration renewal of chemical active pharmaceutical ingredients (APIs) in early November. It is expected that the official announcement will be finalized soon and will facilitate the Drug Master File (DMF) process in China in future. According to the new […]
