New Beginnings, New Horizons: A Strategic Merger for Enhanced Pharmaceutical Solutions
Hangzhou, February 12, 2025 – REACH24H BaiPha […]
Pathway Options for China Drug Master File (DMF) Registration:An Analysis of the Strategic Options
This is the second article in a series focusing on Chin […]
China Drug Master File (DMF) Registration: A Snapshot on Pathways and Requirements
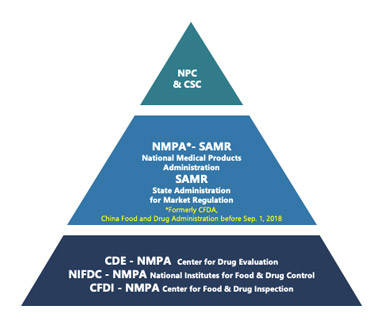
This is the first article in a series focusing on China […]
China’s DMF API License Renewal: Understanding NMPA’s Policy Update
Announcement No. 129 of 2023 was released by the Nation […]
China Drug Master File (DMF): Excipients Exemption List

Did you know that certain pharmaceutical excipients are […]
CHINA CDE EYES MORE DRUG APPROVALS BY 2025

China CDE director vows more support for drug review &a […]
DMF IN CHINA NEW POLICY ON ACTIVE PHARMACEUTICAL INGREDIENTS (API) RENEWAL

NMPA News: China’s National Medical Products Administra […]
